

Meet stringent (and frequently evolving) regulations and standards from the FDA, EMA, and WHO with a comprehensive pharmaceutical safety, compliance, and risk management solution.

The pharmaceutical industry faces the constant challenge of adhering to guidelines, safety protocols, and quality assurance practices across borders. Veriforce simplifies regional and global compliance management so you can focus on research and innovation, without compromising on regulatory obligations.

Use Veriforce to efficiently track and manage third-party compliance worldwide. Our platform is highly configurable, enabling you to input the regulatory requirements relevant to your business and see which contractors are in compliance.
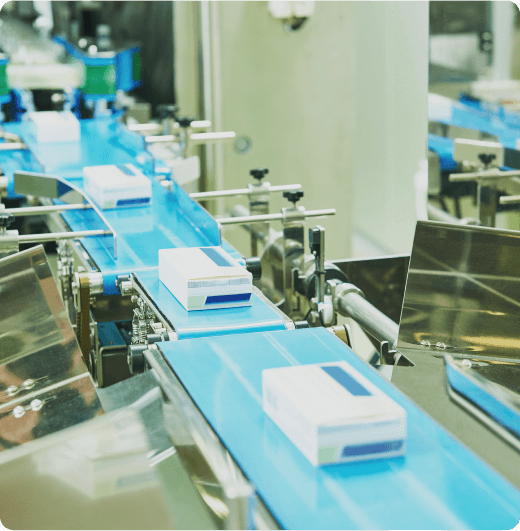
From raw material sourcing to manufacturing, distribution, and storage, every stage of your supply chain is susceptible to risks that could compromise product quality and patient safety. Veriforce gives you complete visibility of contractors’ risk profiles, safety training status, and compliance, so you can identify and solve issues before they impact your business.

Reduce the risk of worker exposure to biohazards, chemical risks, and potent compounds by developing robust pharmaceutical safety protocols. Veriforce gives you tools and resources to deliver safety content, conduct safety assessments, track pharmaceutical safety training progress, and certify employee competence.
We partner with you to configure a solution that meets your unique supply chain needs, locally and around the world. And with global phone, email, or live chat support available in multiple languages, we’re always there when you need us.
Source and manage qualified contractors, suppliers, and vendors.
Equip your workforce with world-class instructor-led and on-demand training.
Build a world-class OQ program to ensure worker competency and compliance defensibility.
Augment your existing resources with auditing, verification, and consulting services.
Gain full visibility into the safety and compliance of all of your subcontractors.
Ensure every on-site worker is qualified, compliant, and ready for work.
Develop a clear mandate and track ESG health across your supply chain.
